The chemical formula of baking powder is sodium hydrogencarbonate NaHCO 3. Originally washing soda was extracted from the ashes of sodium rich plants which is how it got the name soda ash.

What Is The Difference Between Washing Soda And Baking Soda Bren Did
Chemically known as Sodium Carbonate.

Distinguish between baking soda and washing soda by heating. Baking powder NaHCO3 on heating produces carbon dioxide which extinguishes a burning matchstick. This can be shown by following equation. Washing soda is Na2CO3 or Sodium Carbonate while baking soda is Sodium Bicarbonate or NaHCO3.
Mild and does not damage surfaces. Both products can be used to improve liquid laundry performance for cleaner fresher clothes. Both can be synthetically produced by the Solvay process.
The majority of household borax comes from dried lakes in California and Turkey and popular brands like 20 Mule Team Borax advertise it primarily as a laundry booster. 2NaHCO3 Heat Na2CO3 CO2 H2O When washing soda Na2CO3. However washing soda can be naturally derived from plant ashes while baking soda can be collected from nahcolite a naturally occurring mineral.
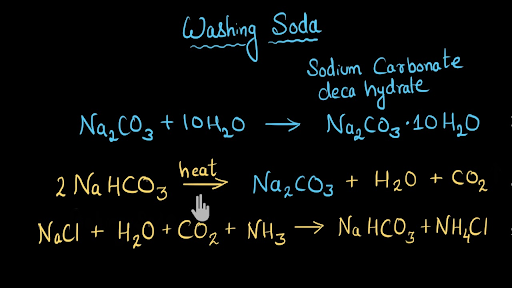
Has a pH level of 81. Washing soda granules dissolve slowly in water and will leave a white residue if not well rinsed. Washing soda also known as soda ash is chemically known as sodium carbonate with the chemical equation Na2CO3.
Washing soda can also be found in the pool department of Wal-Mart or any pool supply outlet under the name pH Plus. When baking powder is heated carbon dioxide is produced. It is a mildly abrasive cleaner that is good for scrubbing.
When baking soda is heated up to high temperatures it breaks down to become washing soda water steam and carbon dioxide. When heated baking soda NaHCO3 aka sodium bicarbonate is converted to washing soda Na2CO3 aka sodium carbonate with the evolution of carbon dioxide gas. The key difference between baking soda and washing soda is that baking soda is sodium bicarbonate whereas washing soda is sodium carbonate.
Unlike baking soda and washing soda borax is mined instead of manufactured. This is what I use for laundry since no stores in my area carry washing soda. Both baking soda and washing soda are salts of sodium.
It is prepared through the Solvay process using sodium chloride ammonia carbon dioxide in water. Baking soda is sodium bicarbonate and is safe for human consumption. Washing soda doesnt do much of anything when heated.
These are useful in different applications. Edible and can be used in baking and cooking. The gas so formed turns lime water milky which confirms the presence of carbon dioxide gas.
Evolution of carbon dioxide can be confirmed by passing the gas into lime water. 10 H2O is heated it does not produce carbon dioxide but it loses water of Crystallisation molecules. The natural mineral form of baking soda is Nahcolite.
Also called cooking soda or bicarbonate of soda. Washing soda is a white-ish powder with larger granules that can be seen and felt. Washing soda is naturally derived from plant ash.
This reaction does not happen when washing soda. It does not decomposes on heating to give out CO2 gas. It also gives sodium carbonate and water vapour.
But youre not likely to notice that evolution if you simply heat the baking soda in the open. Baking soda can be used in baking as a dentifrice and as an antacid washing soda cannot be used in baking on or in the body. Baking powder on heating decomposes to give sodium carbonate water and carbon dioxide according to the following equation NaHCO3 Na2CO3 H2O CO2 Washing soda Na2CO310H2O on the other hand.
2 NaHCO3 Heat Na2CO3 s H2O g CO2 g. On heating it decomposes to give out CO2 gas which can be tested by passing through lime water which turns milky. When Washing soda N a2 C O3.
Whereas that of washing soda is sodium carbonate Na 2 CO 310H 2 O Sodium hydrogencarbonate on heating gives CO 2 gas which will turn lime water milky whereas no such gas is obtained from sodium carbonate. Are solved by group of students and teacher of Class 10 which is also the largest student community of Class 10. The Questions and Answers of How would you distinguish between baking powder and washing soda by heating.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. If limewater turns milky it indicates evolution of carbon dioxide gas. Baking soda gives carbon dioxide and water vapour on heating at even low temperature such as 1000oC.
While baking soda is widely known as a leavening agent washing soda is mainly a laundry booster. Collected from a natural mineral called nahcolite. It can be synthetically produced through the Solvay process using sodium chloride and limestone.
Washing soda is Na2CO310H2O. Molecular formula is NaHCO3. Baking soda is a pure white powder with fine dusty granules that cannot be felt.
Can be applied to the skin. Baking powder contains sodium hydrogen carbonate NaHCO3. Borax has a pH of 95 placing it roughly halfway between baking soda and washing soda on the pH scale.
How To Make Washing Soda If You Can T Find It In Stores
Washing Soda Video Khan Academy
How To Make Washing Soda If You Can T Find It In Stores

What Is The Difference Between Washing Soda And Baking Soda Bren Did

What Is The Difference Between Washing Soda And Baking Soda Bren Did
Is Cooking Soda Same As Baking Soda Quora

How To Make Washing Soda From Baking Soda Wellness Mama

What Is The Difference Between Baking Soda And Washing Soda Baking Soda Cleaning Washing Soda Baking Powder For Cleaning

Post a Comment for "Distinguish Between Baking Soda And Washing Soda By Heating"